
We believe that regrowable materials are essential for a livable future.
That’s why we, Carmen Rommel, Julian Lotz and Vinzenz Nienhaus, founded BIOVOX in 2021. In the meantime, the team surrounding our fully biodegradable bioplastics made from renewable raw materials has grown. With our work, we want to enable a truly sustainable circular economy in the healthcare sector.
You share our vision? We are open to >>new talents.
For a
livable
world.
High-quality.
Sustainable.
Safe.
Developing sustainable products is a complex task, especially when done in a highly regulated environment. Our team brings know-how from plastics technology, medical technology, life cycle assessment (LCA) and product development.
Our goal is to work with you to develop products that are high-quality, sustainable and safe.

Quality
Regulation
Our goal is a sustainable future in which products made from our sustainable, renewable plastics are compatible for both people and the environment.
We continuously focus all our activities on safety and reliability in order to provide our customers with sustainable plastics of the quality essential for safe use in healthcare while ensuring economic success.
This is our contribution to a more livable world.
Medical Grade Plastics (MGP) are polymeric plastics and masterbatches, blends, compounds which [...] are used to produce [...]
>> Medical devices (according to MDR – Regulation (EU) 2017/745)
>> In vitro diagnostics (according to IVDR – Regulation (EU) 2017/746)
>> Primary packaging (according to the guidelines of the European Medicines Agency (EMA), Committee for Medicinal Products for Human Use (CHMP) and Committee for Medicinal Products for Veterinary Use (CVMP))
When supplying medical grade bioplastics to our customers, we maintain a certified quality management system “that shall ensure compliance with [the MDR] in the most effective manner and in a manner that is proportionate to the risk class and the type of device” (MDR, Article 10(9)).
It is certified in accordance with the harmonized ISO 13485 standard to support the fulfilment of the ‘General Safety and Performance Requirements’ (MDR, Annex I).
This ensures that products with reproducible quality properties can be produced at any time using our bioplastics.
In the development and manufacture of Medical Grade Bioplastics, we comply with the requirements of Annex I Sections 10.1 and 10.4.1 of the MDR applicable to materials:
>> The mass proportion of carcinogenic, germ cell mutagenic and reproductive toxic substances of category 1A or 1B according to EU Chemicals Regulation (EC) No. 1272/2008 (CLP) of 28.10.2020 is less than 0.1% in our products.
>> The mass proportion of substances of very high concern published in accordance with Article 59(10) of the REACH Regulation (EC) No. 1907/2006 of 18.12.06 is less than 0.1% in our products.
>> Bioplastics in medical grade quality have been subjected to assessment and evaluation in accordance with ISO 10993 to confirm basic biocompatibility.
According to Regulation (EC) No. 1272/2008 (CLP), the bioplastics of BIOVOX are classified as "non-hazardous". According to Article 31 of the REACH Regulation (EC) No. 1907/2006 of 18.12.06, a safety data sheet is therefore not required for them.
In accordance with Article 32, we will provide you with all information necessary for safe handling, to ensure safety or for your risk management measures. In accordance with Article 32, we will provide you with all information necessary for safe handling, to ensure safety or for your risk management measures. Just contact us!
Sustainability
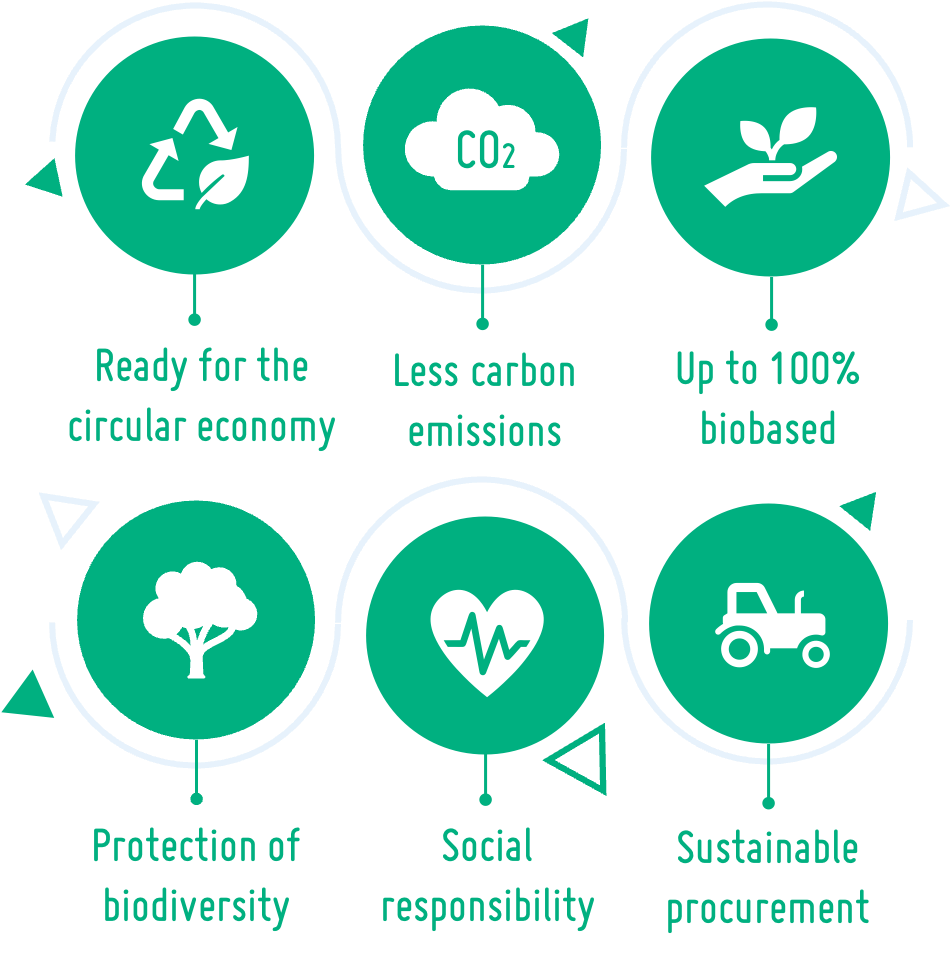
Our bioplastics are up to 100% biobased, ready for circular economy and their low carbon footprint saves up to 85% CO2e compared to conventional medical grade plastics. At the end of their life cycle, our compounds do not release fossil carbon into the atmosphere. All of our materials are recyclable and can therefore be used in a circular economy. Thanks to their suitability for efficient monomer recycling, they can even be used for new medical products in medical grade quality. Throughout the supply chain, we also take care to protect biodiversity and rainforests and live up to our social responsibility.
>> Biobased, recyclable and biodegradable
>> Energy efficient processing
>> Ready for a circular economy
>> Less material needed due to high strength and stiffness
>> No competition to food production
>> Lower carbon footprint than fossil plastics
with similar properties
>> Human rights are respected throughout the whole
supply chain
>> No deforestation for plantations
How much CO2e do BIOVOX bioplastics emit?
The answer depends on which of our product families you choose: MedEco Rigid and MedEco Soft grades have the lowest footprint, whereas our high performing MedEco Perform bioplastics are higher in CO2e emissions. By choosing our most sustainable materials you can save up to 85% CO2e! Additional savings can be realized through lower processing temperatures and materials savings thanks to increased stiffness.
In our Sustainability factsheet you will find the exact numbers.
With the help of our CO2 calculator you can easily calculate the savings potential for your application online.
Ready for a circular economy
The EU requires every industry to move towards a circular economy. BIOVOX MedEco is the best option now, because it has a low carbon footprint, and gets even better with chemical recycling.
A closed loop for MedTech:
Yes, recycling is also possible for medical applications – quality and traceability can be ensured by choosing right recycling process.
Feedstock plants act as a carbon sink, binding CO2 from the atmosphere. This is the same amount of carbon that would be released again when the plastic is incinerated - closing the loop over one year rather than millions of years as with fossil oil: this what is meant by the term defossilization.
Mechanical recycling is out of scope for the very most medical applications: traceability and quality are not achievable at scale. Monomer recycling, however, meets the quality & purity standards for medical grades: The material is absolutely identical to virgin material. A true and compliant same level recycling, saving on carbon and land use.
Only polyesters such as like MedEco can be chemically recycled to monomers. Polyolefins can only be chemically recycled through energy-intensive feedstock recycling (e.g. pyrolysis).
In our Sustainability factsheet you will find a graphical overview of the recycling options, as well as a calculation on co2 savings from recycling.
BIOVOX ensures, that human rights as well as environmental standards are respected along the whole supply chain.
Origin: We are producing in Germany. Our raw materials are sustainably sourced in Europe and Asia.
Biodiversity & Rainforest Protection: All feedstock of BIOVOX bioplastics are certified through ISCC plus & bonsucro, ensuring zero deforestation as well as the protection of biodiversity, soil, water and air.
Social Responsibility: The feedstock certification also includes social and human rights. Covered are good working conditions with safety and health standards as well as communal rights and rural development.
MedEco is a lot – but no competition to food! Currently less than 0,01% of agricultural land is used for bioplastics. In a circular economy, all plastics can be farmed on 2.8% of arable land or 0.31% of total land area. And this calculation does not take into account the use of waste as a feedstock, which is still uncommon for CO2 and cost reasons, but which is possible with our bioplastics. And what if everything was made from bioplastics? Today only 0,08% of the arable land is used to produce bioplastics. All of the world’s plastics can be grown on 13,9% of the arable land. Circular economy requires a maximum of 2,8% of arable land, or 0,31% of the global land area.
In our Sustainability factsheet you will find a graphic showcasing the topic of food competition.
Innovation
& Research
True to Marty McFly's motto „If you put your mind to it, you can accomplish anything.“ we regularly contribute our expertise and curiosity to research projects, test new approaches, and develop solutions with real future potential. Here we provide insights into current projects.
Research and development for premature infants: Premature infants experience a completely different environment in the intensive care unit than in the womb. The SmartNeonatalCare project aims to develop two complementary modules for vital parameter sensing and development-promoting positioning of premature infants. New research and development work involves active physiological vibration programs, patient heat balance, and initial clinical results. The hammock is intended to be a model product for sustainable hospital equipment and to demonstrate the health benefits of sustainable materials.
For more information about the research project, click >>here.
![]()

With strong
Partners &
Customers
life science company
Partner, Networks & Awards
Known from
K-MAG

Julian Lotz reports in an interview with K-MAG how BIOVOX manages to meet the high medical demands with bioplastics.
Medizin&Technik

Medizin&Technik magazine interview with Julian Lotz about biobased solutions for many plastic problems.
CHEManager

The international edition of CHEManager writes about our challenging applications.
Frankfurter Rundschau

BIOVOX impressed in the finals and was voted "Frankfurt Forward Start-up of the Year 2023."














